Mechanical Properties of Iron-Carbon Alloys
Austenite
Austenite as mentioned above is face centred cubic and is not normally present in mild steels at room temperature. The austenite grain size does however influence the grain size of the room temperature ferrite and pearlite.

Micrograph of Austenite, Callister

Alpha Ferrite, Low carbon steel - Callister
Ferrite & Pearlite
Ferrite is body centred cubic, which on its own relatively soft. This is the reason why iron is alloyed with carbon to increase its strength. The presence of carbon strengthens the ferrite by interstitial alloying, but it is the presence of the pearlitic microstructure that is more influential on the mechanical properties, the introduction of Fe3C via pearlite into a steel increases its hardness, tensile and yield strengths at the expense of ductility. Therefore, for the higher carbon content steel, more brittle behaviour is expected and the Charpy transition temperature is increased.
Bainite
Bainite consists of fine thin laths of ferrite imbedded by cementite particles. This appears as needle-like structures under the microscope, though the fine Fe3C are difficult to observe and a Scanning Electron Microscope is preferred for resolution. Bainite is obtained by a medium-level cooling rate. Bainite enjoys increased ductility in comparison to pearlite, while still maintaining high hardness.
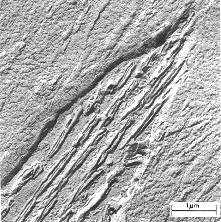

Coarse Pearlite - Callister
Bainite - Callister

Martensite - Callister
Martensite
Martensite is a diffusionless phase transformation of austenite. Martensite formed in steels with a carbon composition <0.6%C is described in Samuels as:
“… [M]artensite has the same composition as the parent austenite from which it forms … The Martensite … grows as parallel arrays, or packets, of basic units which are 0.1 to 0.5 µm thick. Martensite of this morphology is known as lath martensite. The individual laths cannot be generally resolved by optical microscopy, and it is the packets of laths that are visible”
The transformation proliferation takes place extremely quickly. During quenching, the speed of the reaction and the sheer number of dislocations creates quench cracks due to the brittleness of the material. However martensitic steel tends to be very hard and if tempered to between 200-650°C, these internal stresses will dissipate and a strong and hard steel can be produced. However, for the confines of the project, only quenched martensite will be examined as tempering modifies the mechanical properties. Under the microscope, martensitic steel appears in needle patterns contained by grains, as can be seen to the left.